Vacuum Demonstration Pump
The query "Absolute Void" redirects here; for Stanislaw Lem's book, see Absolute Emptiness (book).
Vacuum
(from Latin vacuus - empty) - space free of matter.
In engineering and applied physics, vacuum is understood as a medium consisting of gas at a pressure significantly lower than atmospheric pressure[1]. Vacuum is characterized by the relationship between the free path of gas molecules λ and the characteristic size of the medium d
.
Under d
can be taken the distance between the walls of the vacuum chamber, the diameter of the vacuum pipeline, etc. Depending on the value of the ratio λ/
d
, low ( λ / d ≪ 1 {\displaystyle \lambda /d\ll 1}), medium ( λ / d ∼ 1 {\displaystyle \lambda /d\sim 1} ) and high ( λ / d ≫ 1 {\displaystyle \lambda /d\gg 1} ) vacuum.
Technical vacuum
| This article or section needs revision. Please improve the article in accordance with the rules for writing articles. |
Cross-section of a turbomolecular pump.
In practice, highly rarefied gas is called technical vacuum.
. In macroscopic volumes, an ideal vacuum is unattainable in practice, since at a finite temperature all materials have a non-zero saturated vapor density. In addition, many materials (in particular thick metal, glass and other vessel walls) allow gases to pass through. In microscopic volumes, however, achieving an ideal vacuum is in principle possible.
A measure of the degree of vacuum rarefaction is the free path of gas molecules λ {\displaystyle \lambda } associated with their mutual
collisions in the gas, and the characteristic linear size d {\displaystyle d} of the vessel in which the gas is located.
Strictly speaking, technical vacuum is a gas in a vessel or pipeline with a pressure lower than in the surrounding atmosphere. According to another definition, when gas molecules or atoms stop colliding with each other, and gas-dynamic properties are replaced by viscous ones (at a pressure of about 1 mm Hg), they say that a low vacuum
( λ ≪ d {\displaystyle \lambda \ll d} ; 1016 molecules per 1 cm³).
Usually, between the atmospheric air and the high-vacuum pump there is a so-called fore-vacuum pump, creating a preliminary vacuum, therefore a low vacuum is often called a fore-vacuum
.
With a further decrease in pressure in the chamber, the mean free path λ {\displaystyle \lambda } of gas molecules increases. For λ / d ≫ 1 {\displaystyle \lambda /d\gg 1} gas molecules collide with walls much more often than with each other. In this case, they speak of a high vacuum
(10−5 mm Hg; 1011 molecules per 1 cm³).
Ultra-high vacuum
corresponds to a pressure of 10−9 mmHg. and below. In ultra-high vacuum, for example, experiments are usually carried out using a scanning tunneling microscope. For comparison, the pressure in space is several orders of magnitude lower - 109 molecules per 1 cm³ (a billion molecules per cubic centimeter), while in deep space it can even reach 10-16 mm Hg. and below (1 molecule per 1 cm³)[2].
High vacuum in the microscopic pores of some crystals and in ultrathin capillaries is achieved already at atmospheric pressure, since the diameter of the pore/capillary becomes smaller than the free path of the molecule, which is equal to ~60 nanometers in air under normal conditions [3].
The devices used to achieve and maintain a vacuum are called vacuum pumps. Getters are used to absorb gases and create the required degree of vacuum. The broader term vacuum technology also includes devices for measuring and controlling vacuum, manipulating objects and carrying out technological operations in a vacuum chamber, etc. High-vacuum pumps are complex technical devices. The main types of high-vacuum pumps are diffusion pumps based on the entrainment of residual gas molecules by the working gas flow, getter, ionization pumps based on the introduction of gas molecules into getters (for example, titanium) and cryosorption pumps (mainly to create a forevacuum).
It is worth noting that even in a perfect vacuum at a finite temperature there is always some thermal radiation (gas of photons). Thus, a body placed in an ideal vacuum will sooner or later come into thermal equilibrium with the walls of the vacuum chamber due to the exchange of thermal photons.
Vacuum is a good thermal insulator; The transfer of thermal energy in it occurs only due to thermal radiation, convection and thermal conductivity are excluded. This property is used for thermal insulation in thermoses (Dewar flasks), consisting of a container with double walls, the space between which is evacuated.
Vacuum is widely used in electric vacuum devices - radio tubes (for example, magnetrons of microwave ovens), cathode ray tubes, etc.
How does H2O boil under such conditions?
Any container filled with water always contains air particles. They remain on microscopic cracks on the walls of the container. As the bubbles heat up, they enlarge and become visible to the naked eye, especially on the walls of the vessel and its bottom. Essentially, these are drops of saturated steam dissolved in water.
At a certain stage, the bubbles begin to be pushed out under the influence of the Archimedes force. The water is bubbling, but not yet boiling. This is due to the fact that heating occurs unevenly.
When the temperature at the bottom of the vessel has already reached 100 °C, but not yet at the surface of the water, the force of surface tension and atmospheric pressure prevent particles from leaving the container. They come back, losing their temperature.
When the degree of heating of the surface and bottom layers is equalized, the substance boils . In a vacuum, it is easier for particles to leave the volume of the vessel. Only surface tension prevents this, so boiling begins at a lower temperature.
Physical vacuum
In quantum physics, the physical vacuum is understood as the lowest (ground) energy state of a quantized field, which has zero momentum, angular momentum and other quantum numbers. Moreover, such a state does not necessarily correspond to emptiness: the field in the lowest state can be, for example, the field of quasiparticles in a solid or even in the nucleus of an atom, where the density is extremely high. A physical vacuum is also called a space completely devoid of matter, filled with a field in this state [4] [5]. This state is not absolute emptiness. Quantum field theory states that, in accordance with the uncertainty principle, virtual particles are constantly born and disappear in the physical vacuum: so-called zero-point field oscillations occur. In some specific field theories, the vacuum may have non-trivial topological properties. In theory, several different vacua may exist, differing in energy density or other physical parameters (depending on the hypotheses and theories used). The degeneracy of the vacuum during spontaneous symmetry breaking leads to the existence of a continuous spectrum of vacuum states that differ from each other in the number of Goldstone bosons. Local energy minima at different values of any field, differing in energy from the global minimum, are called false vacua; such states are metastable and tend to decay with the release of energy, passing into a true vacuum or into one of the underlying false vacua.
Some of these field theory predictions have already been successfully confirmed by experiment. Thus, the Casimir effect[6] and the Lamb shift of atomic levels are explained by zero-point oscillations of the electromagnetic field in the physical vacuum. Modern physical theories are based on some other ideas about vacuum. For example, the existence of several vacuum states (the false vacua mentioned above) is one of the main foundations of the Big Bang inflationary theory.
False vacuum
Scalar field φ
in a state of false vacuum.
The energy E
is higher than in the true vacuum state (ground state), but the potential barrier prevents the field from transitioning. Thus, the transition is possible only at high field energies or through quantum mechanical tunneling
Main article: False vacuum
False vacuum
- a state in quantum field theory that is not a state with a globally minimum energy, but corresponds to its local minimum. This state is stable for a certain time (metastable), but can “tunnel” into a state of true vacuum.
Einstein's vacuum
Main article: Einsteinian vacuum
Einstein's vacuum
- a sometimes used name for solutions of Einstein's equations in general relativity for empty, matter-free space-time.
Synonym: Einstein space
.
Einstein's equations relate the space-time metric (metric tensor g
μν) with the energy-momentum tensor.
In general, they are written as G μ ν + Λ g μ ν = 8 π G c 4 T μ ν , {\displaystyle G_{\mu \nu }+\Lambda g_{\mu \nu }={8\pi G \over c^{4}}T_{\mu \nu },}
where the Einstein tensor G
μν is a definite function of the metric tensor and its partial derivatives,
R
is the scalar curvature, Λ is the cosmological constant,
T
μν is the energy-momentum tensor of matter, π is the number pi,
c
is the speed of light in vacuum,
G
is Newton’s gravitational constant.
Vacuum solutions of these equations are obtained in the absence of matter, that is, when the energy-momentum tensor is identically equal to zero in the considered region of space-time: T
μν = 0. Often the lambda term is also taken to be zero, especially when studying local (non-cosmological) solutions.
However, when considering vacuum solutions with a nonzero lambda term ( lambda vacuum
), important cosmological models arise such as the De Sitter model (Λ > 0) and the anti-De Sitter model (Λ < 0).
The trivial vacuum solution to Einstein's equations is the flat Minkowski space, that is, the metric considered in the special theory of relativity.
Other vacuum solutions of Einstein's equations include, but are not limited to, the following cases:
- Milne cosmological model (a special case of the Friedmann metric with zero energy density)
- Schwarzschild metric describing the geometry around a spherically symmetric mass
- Kerr metric describing the geometry around a rotating mass
- Plane gravitational wave (and other wave solutions)
Space
Main article: Outer space
Outer space is not a perfect vacuum; rarefied plasma is filled with charged particles, electromagnetic fields, and sometimes stars
Outer space has very low density and pressure and is the best approximation of a physical vacuum. But the vacuum of space is not truly perfect; even in interstellar space there are a few hydrogen atoms per cubic centimeter.
Stars, planets and satellites hold their atmospheres together by gravity, and as such the atmosphere has no clearly defined boundary: the density of atmospheric gas simply decreases with distance from the object. The Earth's atmospheric pressure drops to about 3.2 × 10−2 Pa per 100 km altitude - at the so-called Karman line, which is the general definition of the boundary with outer space. Beyond this line, the isotropic pressure of the gas quickly becomes negligible compared to the radiation pressure from the Sun and the dynamic pressure of the solar wind, so the pressure determination becomes difficult to interpret. The thermosphere in this range has large gradients of pressure, temperature and composition, and is highly variable due to space weather.
The atmospheric density during the first few hundred kilometers above the Karman line is still sufficient to provide significant resistance to the movement of artificial Earth satellites. Most satellites operate in this region, called low-Earth orbit, and must fire their engines every few days to maintain a stable orbit.
Outer space is filled with a large number of photons, the so-called relic radiation, as well as a large number of relic neutrinos, which are not yet detectable. The current temperature of these emissions is about 3 K, or −270 °C or −454° Fahrenheit.
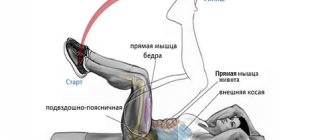
Who should not do a vacuum?
Experts advise paying special attention to this point and adhering to the following recommendations. For those who have problems with the kidneys or cardiovascular system, it is best to consult with specialists before starting such training.
Also a special point for women that is important to pay attention to. During critical days, such exercises are contraindicated. The same applies to those women who are expecting a child. Moreover, it is interesting that immediately after childbirth such exercises are allowed, and they do not in any way affect the amount of milk in a young mother.
Particular attention should be paid to those who are bothered by abdominal pain. Also, if you have an ulcer, this exercise is contraindicated until complete recovery, otherwise the health situation may worsen.
Most people who follow a healthy lifestyle often use the vacuum exercise. It is extremely popular among those who want to quickly achieve a flat stomach and narrow waist. And the most important thing is motivation. For those who want to achieve something, there are no barriers.
History of vacuum research
Mercury vacuum barometer of Evangelista Torricelli, the scientist who first created a vacuum in the laboratory.
Above the surface of the mercury in the upper part of the sealed tube is a “Torricelli void” (a vacuum containing mercury vapor under saturation pressure at a given temperature). The idea of vacuum (emptiness) has been the subject of debate since the times of ancient Greek and Roman philosophers. Atomists - Leucippus (c. 500 BC), Democritus (c. 460-370 BC), Epicurus (341-270 BC), Lucretius (c. 99 -55 BC) and their followers assumed that everything that exists is atoms and the void between them, and without a vacuum there would be no movement, atoms could not move if there was no empty space between them. Strato (c. 270 BC) and many philosophers in later times believed that the void could be "solid" ( vacuum coacervatum
) and “scattered” (in the spaces between particles of matter,
vacuum disseminatum
).
On the contrary, Aristotle (384-322 BC) and a number of other philosophers believed that “nature abhors a vacuum.” The concept of “fear of emptiness” ( horror vacui)
), which originated even before Aristotle, with Empedocles (c. 490-430 BC) and other philosophers of the Ionian school, became dominant in the philosophical thought of Medieval Europe and acquired religious and mystical features.
Some prerequisites for the empirical study of vacuum existed in antiquity. Ancient Greek mechanics created various technical devices based on air rarefaction. For example, water pumps, operating by creating a vacuum under a piston, were known back in the time of Aristotle. A drawing of a fire pump, invented by the “father of pneumatics” Ctesibius (c. 150 BC), has survived to this day. Water pumps of this type were actually the prototypes of the vacuum piston pump, which appeared almost two millennia later. A student of Ctesibius, Heron of Alexandria, developed a piston syringe for drawing out pus, which is also essentially a vacuum device.
The empirical study of vacuum began only in the 17th century, with the end of the Renaissance and the beginning of the scientific revolution of modern times. By this time, it had long been known that suction pumps could lift water to a height of no more than 10 meters. For example, in the treatise “On Mining” by George Agricola (1494-1555), there is an image of a chain of water pumps for pumping water from a mine.
Galileo, in his Discourses and Mathematical Proofs of the Two New Sciences [7] (1638), a book that completed the destruction of Aristotelian physics, pointed out, referring to practice, that the height to which suction pumps raise water is always the same - about 18 cubits. In this book, in particular, he describes, in fact, a vacuum device with a piston, necessary for comparing the tensile strength of water and a solid, although he explains the tensile resistance characteristic of solids and liquids by the fear of emptiness, suggesting the existence of tiny empty pores between the particles of the substance , expanding when stretched.
Under the influence of Galileo’s treatise, which pointed out the limitations of the “fear of emptiness,” in 1639-1643. Gasparo Berti built a device (in later terminology, a barometric water pipe) on the facade of his house in Rome, which can be considered the first installation for the physical study of vacuum. In the upper, glass closed part of the pipe, more than 10 m high, above the water column, balanced by atmospheric pressure, an empty space was found (in fact, it was filled with water vapor under pressure equal to the elasticity of water vapor at ambient temperature, as well as dissolved water released from the water air, that is, the pressure in the cavity was about 0.1 atmosphere). Emanuel Magnano secured a bell and a hammer in this cavity. Using a magnet on the hammer, he struck the bell with the hammer. As a result of this first-ever experiment in a vacuum (more precisely, in a rarefied gas), it was discovered that the sound of the bell was muffled[8].
The scientist Raffaello Maggiotti[9] (1597-1656) from Rome reported the experiments of Berti and Magnano to Galileo’s student, the Florentine Evangelista Torricelli. At the same time, Maggiotti expressed the idea that a denser liquid would stop at a lower level [10]. In 1644, Torricelli (with the help of Vincenzo Viviani, another student of Galileo) managed to create the first vacuum chamber. His work related to theories of atmospheric pressure served as the basis for additional experimental techniques. A Torricelli vacuum (Torricelli vacuum) is achieved by filling a long glass tube, sealed at one end, with mercury, and then inverting it so that the open end of the tube is below the surface of the mercury in a wider open vessel.[11] Mercury will flow out of the tube until the gravity of the mercury column is compensated by atmospheric pressure. A vacuum is formed in the mercury-free space at the upper, sealed end of the tube. This method underlies the operation of a mercury barometer. At standard atmospheric pressure, the height of the mercury column balanced by atmospheric pressure is 760 mm.
Von Guericke's vacuum pump and the Magdeburg hemispheres in the Deutsche Museum in Munich
Around 1650, the German scientist Otto von Guericke invented the first vacuum pump (a piston cylinder with a water seal), which made it possible to easily pump air out of sealed containers and experiment with vacuum[12]. Pump named by the author antlia pneumatica
, was still very far from perfect and required at least three people to manipulate the piston and taps, immersed in water, to better isolate the resulting void from the outside air. However, with his help, Guericke was able to demonstrate many of the properties of vacuum, in particular, by performing the famous experiment with the Magdeburg hemispheres. Guericke also created a water barometer, similar in principle to Torricelli's mercury barometer, although due to the lower density of water compared to mercury, the height of the water column balancing atmospheric pressure is 13.6 times greater - about 10 meters. Guericke was the first to discover that a vacuum does not conduct sound and that combustion stops in it[13].
Guericke's vacuum pump was significantly improved by Robert Boyle, which allowed him to perform a series of experiments to determine the properties of vacuum and its effect on various objects. Boyle discovered that in a vacuum small animals die, fires go out, and smoke sinks down (and is therefore just as affected by gravity as other bodies). Boyle also found out that the rise of liquid in capillaries also occurs in a vacuum, and thereby refuted the then prevailing opinion that air pressure was involved in this phenomenon. On the contrary, the flow of liquid through the siphon in a vacuum stopped, which proved that this phenomenon was caused by atmospheric pressure. He showed that during chemical reactions (such as slaking lime), as well as during mutual friction of bodies, heat is released in a vacuum.
Why can it boil at negative temperatures?
When the environment is rarefied, water boils earlier. Boiling will begin as soon as the vacuum reaches a value at which the boiling point becomes less than the ambient temperature.
The table below shows rounded data on the dependence of boiling point on pressure.
| Pressure, Pa | Boiling point of water | ||
| °C | °F | °K | |
| 101 325 | 100 | 212 | 373 |
| 84 660 | 95 | 205 | 368 |
| 70 060 | 90 | 194 | 363 |
| 47 340 | 80 | 176 | 353 |
| 31 550 | 70 | 158 | 343 |
| 19 900 | 60 | 140 | 333 |
| 12 300 | 50 | 122 | 323 |
| 7 350 | 40 | 104 | 313 |
| 4 230 | 30 | 86 | 303 |
| 3 380 | 27 | 80 | 300 |
| 3 048 | 25 | 76 | 298 |
| 2 710 | 22 | 72 | 295 |
| 2 370 | 20 | 69 | 293 |
| 2 030 | 18 | 64 | 291 |
| 1 670 | 15 | 59 | 288 |
| 1 350 | 12 | 53 | 285 |
| 1 010 | 7 | 45 | 280 |
| 605 | 0 | 32 | 273 |
| 340 | -6 | 21 | 267 |
| 170 | -15 | 6 | 258 |
| 35 | -31 | -24 | 242 |
| 0,16 | -47 | -35 | 226 |
| 0,3 | -51 | -60 | 222 |
| 0,03 | -56 | -70 | 217 |
The water gives off steam and cools down. It condenses and returns back to its liquid state. With further pumping of air, the vacuum becomes such that H2O instantly boils .
The temperature drops to negative, water vapor crystallizes, forming ice. Since this is accompanied by an increase in volume, ice formation is prevented by external pressure.
The smaller it is, the earlier ice forms. Therefore, even at low rarefaction, water will inevitably turn into steam, then into ice.
Effect on people and animals
People and animals exposed to vacuum lose consciousness within seconds and die from hypoxia within minutes, but these symptoms are generally not similar to those shown in popular culture and media. The decrease in pressure lowers the boiling point at which blood and other biological fluids must boil, but the elastic pressure of the blood vessels does not allow the blood to reach the boiling point of 37 ° C [14]. Although the blood does not boil, the effect of gas bubbles forming in it and other body fluids at low pressures, known as ebullism (aerial emphysema), is a serious problem. The gas can inflate the body to twice its normal size, but the tissues are elastic enough to prevent tearing.[15] Edema and ebullism can be prevented by wearing a special flight suit. Shuttle astronauts wore special elastic clothing called the Crew Altitude Protection Suit.
(CAPS), which prevents ebullism at pressures greater than 2 kPa (15 mmHg)[16]. The rapid evaporation of water cools the skin and mucous membranes to 0 °C, especially in the mouth, but this does not pose a great danger.
Experiments on animals show that after 90 seconds of the body being in a vacuum, a rapid and complete recovery of the body usually occurs, but a longer stay in a vacuum is fatal and resuscitation is useless[17]. There is only limited data on the effects of vacuum on humans (usually it has occurred in accidents), but it is consistent with data obtained from animal experiments. Limbs can remain in a vacuum much longer if breathing is not impaired[18]. Robert Boyle was the first to show that vacuum was lethal to small animals in 1660.
The essence of sous-vide technology
Sous-vide is a method of cooking food in hermetically sealed plastic bags at low temperatures for long periods of time.
The two main differences between sous-vide and traditional cooking methods are that:
- the raw product is packaged in plastic bags and the food is cooked using precisely controlled heat;
- food is cooked at low temperatures in the range of 58–64.5 °C, which allows you to achieve unique taste properties, softness and aroma.
Vacuum packaging prevents the loss of aromatic volatile substances and moisture during cooking, and also prevents the appearance of rancid tastes that are not typical for food. All this contributes to the preparation of flavorful and nutritious food. Vacuum packaging also reduces the growth of aerobic bacteria and promotes efficient transfer of thermal energy from the water (or steam) to the food.
Accurate temperature control is essential when cooking fish, meat and poultry. Consider the problem of preparing a thick-cut steak cooked until the blood clots. Grill steak over 500°C until center reaches 50°C. This will cause the center portion of the steak to be overcooked. To avoid this problem, first sear the steak on one side in a pan, turn it over and place the pan in a combi oven at 135°C until the center reaches 55°C. When cooking sous-vide, the steak is sealed in a plastic bag, cooked in a water bath at 55 °C for several hours, and then fried in a smoking hot pan or using a torch; As a result, we get an evenly fried steak with blood and a thick crust. What's more, the flavorful steak can be cooked (very safely) in a 55°C water bath for 24 hours, resulting in a tender, rare steak. In this case, unlike the first option, its inner part will remain with blood and will not be overcooked.
Sous-vide technology usually consists of three stages: preparing the product for packaging, cooking and post-cooking. In almost all cases, cooking in a water bath or in a combi oven is optimal. When using a combi oven, you can cook more food, but the oven does not heat evenly enough, and the error in the oven thermostat will also not allow you to achieve the desired result. Experts have proven that none of the tested convection ovens heat food evenly when fully loaded. Heating of the package occurs much slower (in standard mode), 70-200% longer in a sous-vide thermostat. It is believed that this is a result of the relatively poor distribution of steam at temperatures below 100 °C and the dependence of the furnace on the amount of steam in the heat exchange medium. Unlike a combi oven, a sous-vide thermostat heats a water bath very evenly and typically provides an error of less than 0.05°C.
It is important to note that sous-vide cooking is cooking in which the cook no longer has any influence on the process. All those standards for laying ingredients, spices and seasonings that were maintained at the beginning of the process can no longer be changed during its course. Obviously, the cook cannot help but taste the dish and influence the process when the package is already sealed and placed in a water bath. That is why the process of preparing ingredients for putting in a bag must be done very carefully.
Stowing products and preparing ingredients
With sous-vide cooking, marinating food is a much more involved process than with conventional cooking. While many herbs and spices work well in a dish, others are more intense and can easily overpower a dish with their aroma and flavor. In addition, some products (such as carrots, onions, celery, bell peppers, etc.) will not soften or add flavor to the dish as much as they do during normal cooking, since the “cooking” temperature is too low for this to happen. to soften starch or cell membranes. In fact, unlike meat, most vegetables require higher temperatures (80 to 85 °C) and therefore must be cooked separately. And finally, fresh garlic leads to very unpleasant and pronounced results, so it is recommended to replace it with a dry mixture and then in very small quantities.
With longer cooking times (more than a few hours), we have noted that olive oil produces off-flavors, reminiscent of metallic or bloody flavors. When olive oil is produced, it is not heated or refined, meaning that some oils will break down even at low temperatures. A simple solution to this situation is to use grapeseed oil or any other processed oils designed for longer cooking; Olive oil can be used for seasoning or sauces after cooking.
It is recommended to salt and fluff the meat before vacuum packing it.
Most marinades are acidic and contain either vinegar, wine and fruit juice, or kefir and yogurt. Of all these ingredients, only wine can cause significant problems when cooking sous-vide. If the alcohol is not evaporated before marinating, some of it may change from liquid to vapor while in the bag, causing the meat to cook unevenly. Simply evaporating the alcohol before marinating will solve this problem.
Mechanical tenderizing using Jacquard knives has become a fairly common method today. A jacquard is a set of thin blades that pierce the meat and cut out some of the internal tissue. Jacquard generally does not leave visible marks on the meat and is often used in steakhouse-style restaurants. By cutting through a lot of the inner tissues that would normally interact with the heat and squeeze out the juices, you can slightly reduce moisture loss during cooking. For example, when the shoulder was cooked at 55°C for 24 hours, the Jacquard-precooked steak lost 18.8% of its weight, compared to the regular steak, which lost 19.9%. In general, the longer a piece of meat is cooked at a given temperature, the more weight it loses. However, this additional weight loss is balanced by increased softness due to the dissolved collagen turning into gelatin.
Brining is becoming more and more popular in modern cooking, especially when preparing pork and poultry. Typically, the meat is placed in a 3-10% (30-10 grams per liter) salt solution for several hours, then washed and cooked as usual. Brining has two effects: it dissolves some of the tiny muscle tissue structures so that they cannot coagulate, and it allows the brined meat to absorb 10-25% of its weight in moisture (the brine can be flavored with aromatic herbs and spices). Since meat still loses about 20% of its weight during cooking, the net effect would be to lose only 0-12% of its original weight.
Preparation
There are two schools of thought when it comes to sous-vide cooking: the temperature of the water bath should be slightly higher or significantly higher than the desired final internal temperature of the product. While the second option is closer to traditional cooking methods and has long been widely used in gastronomy, the first option has several significant advantages over the second. We recommend working at a temperature 0.5-1°C higher than the desired final internal temperature of the food.
When cooking in a water bath at a temperature significantly higher than the desired final internal temperature of the product, it should be removed from the bath as soon as it reaches the desired temperature to prevent overcooking.
Conversely, bain-marie cooking at just above the product's desired final internal temperature means that the product can remain in the bain-marie indefinitely without being overcooked. Thus, food can be pasteurized in the same water bath in which it was cooked. Although the cooking time is thus significantly increased, unlike traditional cooking methods, the meat comes to temperature surprisingly quickly, since the thermal conductivity of water is 23 times higher than the thermal conductivity of air.
Effect of Heat on Meat
Muscle meat, as we mentioned in previous chapters, contains 75% water, 20% protein, 5% fat and other substances. Proteins in meat can be divided into 3 groups: myofibrillar (50–55%), sarcoplasmic (30–34%) and connective tissue (10–15%). Myofibrillar proteins (mainly myosin and actin) and connective tissue proteins (mainly collagen) interact when heated, while sarcoplasmic proteins expand when heated. These changes are usually called denaturation.
During heating, muscle fibers contract transversely and longitudinally, sarcoplasmic proteins accumulate and gel, and connective tissue contracts and dissolves. Muscle fibers begin to contract at temperatures of 35–40°C and contraction increases almost linearly at temperatures up to 80°C. The accumulation and gelation of sarcoplasmic proteins begins at 40 °C and ends at 60 °C. Connective fibers begin to contract at 60°C, but interact more intensely at temperatures above 65°C.
The water-holding capacity of all muscle meat is regulated by the contraction and swelling of myofibrillar fibers. About 80% of the water in muscle meat is held in the myofibrils between the thick (myosin) and thin (actin) fibers. At temperatures between 40 and 60 °C, the muscle fiber contracts along the surface and expands the space between the fibers. Then, at temperatures above 60–65 °C, the muscle fiber contracts transversely, resulting in significant water loss; the magnitude of such losses increases with temperature.
When cooking tender meats, we just need to concentrate on the temperature and, when pasteurizing, maintain it for a certain period of time (see table 1).
Attention! Cooking time depends on the thickness of the meat: doubling the thickness of the meat increases the cooking time of the meat by 4 times!
Table 1 – Temperatures appropriate for cooking rare, medium, or medium-rare meat or fish
Cooling for later use
In the food industry, sous-vide is used to increase the shelf life of cooked food. Once pasteurized, the food is quickly cooled in its vacuum sealed bag and frozen (or refrigerated) until needed. Before cooking is complete, the food is heated in a water bath at or below the temperature at which it was cooked. Usually the meat is heated in a water bath at a temperature of 55 °C.
The danger of cooking and refrigerating the product is that pasteurization does not reduce the number of pathogenic spores to a safe level. If food is not cooled quickly enough or is frozen for too long, the number of pathogenic spores can increase to dangerous levels. Cooling methods are given in the previous chapter.
Completion of cooking (pre-cooking)
Because sous-vide is a very controlled and precise cooking method, most foods cooked sous-vide appear cooked.
Thus, fish, shellfish, eggs and skinless poultry can be served as is. But steaks and pork chops require browning and sauce. Browning meat is a very popular method as the incipient Maillard reaction adds a nice color and flavor.
Maillard reaction
As we already know, the flavor of cooked meat comes from the Maillard reaction and the thermal (and oxidative) degradation of lipids (fats). The reaction promotes the appearance of aromas of cooking, frying, and pungency in muscle tissue. The Maillard reaction can be increased by adding a reducing sugar (glucose, fructose, or lactose), increasing the pH (for example, by adding a pinch of baking soda), or increasing the temperature. Even small increases in pH greatly increase the Maillard reaction and result in sweeter flavors in grilled meats. Adding a drop of glucose (such as corn syrup) also enhances the Maillard reaction and improves flavor.
Measurement
Main article: Pressure measurement
The degree of vacuum is determined by the amount of substance remaining in the system. Vacuum is primarily determined by absolute pressure, and full characterization requires additional parameters such as temperature and chemical composition. One of the most important parameters is the mean free path (MFP) of the residual gases, which indicates the average distance a particle travels during its free path from one collision to the next. If the gas density decreases, the MFP increases. The MFP in air at atmospheric pressure is very short, about 70 nm, and at 100 mPa (~1 × 10−3 torr) the MFP of air is about 100 mm. The properties of a rarefied gas change greatly when the mean free path becomes comparable to the size of the vessel in which the gas is located.
Vacuum is divided into ranges according to the technology required to achieve or measure it. These ranges do not have universally accepted definitions, but a typical distribution looks like this[19][20]:
| Pressure (mmHg) | Pressure () | |
| Atmosphere pressure | 760 | 1,013×10+5 |
| Low vacuum | from 760 to 25 | from 1×10+5 to 3.3×10+3 |
| Medium vacuum | from 25 to 1×10−3 | from 3.3×10+3 to 1.3×10−1 |
| High vacuum | from 1×10−3 to 1×10−9 | from 1.3×10−1 to 1.3×10−7 |
| Ultra high vacuum | from 1×10−9 to 1×10−12 | from 1.3×10−7 to 1.3×10−10 |
| Extreme Vacuum | <1×10−12 | <1,3×10−10 |
| Space | from 1×10−6 to <3×10−17 | from 1.3×10−4 to <1.3×10−15 |
| Absolute vacuum | 0 | 0 |
apply to non-coindensing gas and steam. Gas pressure on a bounding surface is the ratio of the normal component of the force acting from the gas on the bounding surface to the area of this surface. Pressure at a certain point in gas space is the ratio of the rate of transfer of the normal component of momentum, which is determined by the movement of molecules in both directions through a region on an imaginary plane passing through the point in question, to the area of this region (in the presence of a flow of gas molecules, indicate the orientation of the plane relative to the vector this stream). Pumping is a reduction in the molecular concentration of a gas using devices that remove or absorb gas. Pumping time is the time required to reduce the pressure in the pumped vessel to a certain value by a pump of a particular type or type. Residual gas is the gas remaining in the vacuum system after pumping. Ultimate residual pressure is the lowest pressure that can be achieved under certain conditions when using specific pumping devices. Fore-vacuum is a vacuum created by a lower vacuum pump when several pumps operate in series. Absolute gas pressure is the gas pressure measured from zero. Atmospheric pressure is the absolute pressure of the atmosphere. The normal state of a gas is the state of a gas under normal conditions: pressure 101,325 Pa and temperature 273 K. Rarefied gas is a gas whose molecular concentration is less than its concentration under normal conditions. Partial pressure is the pressure of a certain component of a gas mixture. Total pressure is the sum of the partial pressures of the components of the gas mixture. The amount of gas is the product of the volume occupied by the gas and its pressure. Steam is a gas whose temperature is below the critical temperature (a gas that can be converted into the condensed phase only by increasing pressure). Saturated steam is steam that is in thermodynamic equilibrium with one of the condensed phases of the substance in question at a given temperature. Unsaturated vapor is steam whose pressure is less than the saturated vapor pressure of a given substance at the same temperature. The degree of saturation is the ratio of vapor pressure to saturated vapor pressure. Molecular concentration is the number of gas molecules per unit volume. Gas density is the mass per unit volume of gas. Gas density per unit pressure is the ratio of gas density to its pressure. The free path length of a molecule is the length of a molecule's path between two successive collisions with other molecules. The average free path length of a molecule is the arithmetic average of the distances. which a molecule passes between two successive collisions with other molecules. The number of collisions per unit time is the arithmetic average of collisions of a molecule per unit time with other molecules. The effective free path length of molecules is the ratio of the average free path length of molecules to the probability of a certain process (phenomenon) as a result of one collision (the probability of a certain process is understood as the ratio of the number of collisions in which this process occurs, for example ionization, to the number of collisions over a sufficiently large period of time ). Gas diffusion is the movement of gas in another medium under the influence of a concentration gradient. The diLfushi coefficient is the ratio of the absolute flow rate of molecules through a unit surface area to the concentration gradient, provided that the surface is normal to the gradient. Gas flow is the movement of gas in a pipeline under the influence of pressure or temperature differences at its ends. Viscous flow is the flow of gas in a channel under conditions where the average free path length of molecules is very small compared to the smallest internal transverse dimension of the channel. Laminar flow is a gas flow characterized by the absence of mixing between adjacent layers. Turbulent flow is a gas flow in which the molecules are completely there are unsteady, disorderly movements along complex trajectories. Poieil flow is a laminar viscous flow in a long pipe of circular cross-section. Molecular flow is the flow of gas in a channel under conditions where the average free path length of molecules significantly exceeds the internal transverse size of the channel. Molecular-viscosity flow is the flow of gas in a channel under conditions intermediate between the conditions of laminar, viscous and molecular flows. Effusion flow is the flow of gas through a hole under conditions where the largest size of the hole is less than the average free path length of the molecules. Temperature transpiration is the flow of gas between connected vessels under the influence of the temperature difference between the vessels, which results in the formation of a “pressure gradient.” Molecular flow is the number of molecules passing through a certain cross section per unit time. The resulting molecular flux is the ratio of the molecular flux determined by the difference between the number of molecules crossing a surface in a given time interval in a given direction and the number of molecules crossing that surface in the opposite direction by that time. Molecular flux density is the ratio of the resulting molecular flux to the surface area that it intersects. Gas mass flow is the mass of gas crossing a certain surface per unit time. Volumetric gas flow is the volume of gas at specified temperatures and pressures crossing a specified surface per unit time. Molar flow of a gas is the number of moles of a given gas crossing a specific surface per unit time. Conductivity is the ratio of the flow to the difference in average pressures in two specified sections of the channel or on both sides of the channel opening, assuming isothermal equilibrium. Molecular conductivity is the ratio of the resulting flow of molecules to the difference in the average numbers of molecules per unit volume on both sides of an opening or in two cross sections of a channel. Resistance is the reciprocal of conductivity. Sorption is the absorption of gas or vapor by a solid or liquid (sorbent). Adsorption is the absorption of gas or vapor (adsorbate) by the surface of a solid or liquid (adsorbent). Absorption is the absorption of a gas (absorbate) by a volume of solid or liquid (absorbent). Physical sorption is sorption under the influence of physical forces, in which chemical bonds are not formed. Chemisorption is sorption in which chemical bonds are formed. The accommodation coefficient is the ratio of the average energy actually transferred to the surface by the incident particles to the average energy that could be transferred to the surface by the incident particles if they were torn away from the surface after reaching complete thermal equilibrium with it. Collision frequency is the ratio of the number of molecules colliding with a surface in a given time interval to that time interval and the surface area. Condensation rate is the number of molecules condensing per unit surface area per unit time. The adhesion rate is the number of molecules sorbed on a unit surface area per unit time. The probability of adhesion is the ratio of the rate of adhesion to the frequency of molecular collisions. Retention time is the average time during which molecules are retained on a surface in a state of sorption. Migration is the movement of molecules on a surface. Desorption is the release of gases or vapors sorbed by any material. Gas emission is the spontaneous release of gas from a material into a vacuum. Dehydration is the forced removal of gas from a material. Evaporation rate is the number of molecules of a substance evaporating from a unit surface area per unit time. The permeability of a solid partition is the ratio of the gas flow through the partition to the flow through the same section in the absence of the partition, which is the sum of the pressures on both sides of the partition and its structure. Permeability coefficient is the ratio of the product of permeability and the thickness of the partition to its area. Leakage is the penetration of gas from the environment into the pumped out (evacuated) vessel. Vacuum system ■ its elements Vacuum system is a set of interconnected devices for creating, increasing and maintaining vacuum, instruments for vacuum measurements, as well as evacuated vessels and vacuum pipelines connecting them (devices that ensure the operation of a vacuum system include, for example, an electric motor, batteries, ovens). Vacuum installation - an installation consisting of a vacuum system and devices that ensure its operation. Vacuum unit is a vacuum installation, structurally designed as a single unit. Pumping station is a vacuum installation designed for pumping, filling and training products. An element of a vacuum system is a device, assembly unit or part designed to perform certain functions in a vacuum system (for example, vacuum pump, pressure transducer, trap, etc.). Conditional bore is the diameter of the flow section of a vacuum system element, which determines the connecting dimensions according to current standards. An evacuated vessel is a vessel in which a vacuum is created. A safety cylinder is a vessel designed to protect the elements of the vacuum system from foreign bodies entering them. An equalizing vacuum cylinder is a vessel designed to equalize pressure fluctuations in a vacuum system. A vacuum protective device is an element of a vacuum system designed to quickly separate the section of the vacuum system where a breakthrough of atmospheric air occurred from the rest of it. Vacuum pipeline is a pipeline through which rarefied gas moves in a vacuum system. Fore-vacuum pipeline - a vacuum pipeline used for connection to the fore-vacuum pump. Bypass pipeline is a vacuum pipeline designed to pump out a vessel, bypassing a high-vacuum pump. A comb is a tube with a number of extensions, pre-diastolic for connecting several pumped out vessels. A vacuum sluice is a device for introducing or removing various objects from a vacuum system without breaking the vacuum. Vacuum lubricant is a sealant in the form of a viscous substance with low vapor pressure, used in moving joints of vacuum systems. Vacuum putty - sealant In the form of a plastic substance with low vapor pressure, used in collapsible fixed joints of a vacuum system, as well as to eliminate leaks. A polished section is a combination of two ground surfaces that provides a hermetically sealed connection between the elements of a vacuum system (the section can be movable or stationary). A vacuum hose is a flexible tube that does not shrink under the influence of atmospheric pressure, which serves to connect individual elements of the vacuum system. A vacuum valve is a device that allows you to regulate or completely shut off the flow of gas into a vacuum system. Vacuum seal is a vacuum valve that allows you to connect and disconnect elements of a vacuum system. Vacuum leak is an inlet vacuum valve designed for inlet and regulation of small gas flows. Inlet vacuum valve is a vacuum valve designed to admit air or gas into a vacuum system. Vacuum input is a device for transferring1 mechanical or electrical energy into a vacuum vessel. Exhaust filter is a device located on the outlet side of a vacuum pump with an oil seal and designed to remove oil mist from the exhaust gas. A trap is a device in which the partial pressure of the components of a gas-vapor mixture is reduced mechanically, physically or chemically and the penetration of vapors or gases from one part of the pumping system to another is reduced. A condensation vacuum trap is a trap whose action is based on the condensation of vapors and gases on internal cooled surfaces (according to the cooling method, water, nitrogen-water, freov, thermoelectric and other condensation traps are distinguished; according to their location in the vacuum system, condensation forevacuum and high-vacuum traps are distinguished). A sorption vacuum trap is a trap whose action is based on the sorption of vapors and gases by the surface of a porous sorbent (based on the sorbent used, zeolent, carbon, silica gel and other sorption traps are distinguished; according to their location in the vacuum system, sorption forevacuum and high-vacuum traps are distinguished; the sorbent can be cooled with water, liquid nitrogen, etc.). An ion vacuum trap is a trap in which nx ionization is used to remove certain components of a gas mixture. An oil separator is a device designed to separate gas from oil. An oil purifier is a device designed to remove contaminants from vacuum oil. Equipment for obtaining and maintaining a vacuum A vacuum pump is a device designed to create, increase and (or) maintain a vacuum. Low-vacuum pump is a vacuum pump designed to reduce the pressure in the pumped volume, starting from atmospheric pressure. A high-vacuum pump is a vacuum pump operating at the lowest pressure stage of an exhaust system that consists of two or more pumps connected in series. A fore-vacuum pump is a vacuum pump designed to maintain the outlet pressure of another pump. Booster vacuum pump is a vacuum pump installed between the fore-vacuum and high-vacuum pumps to increase the pumping speed of the pump system at medium vacuum or to optimize the pressure in the pumping system and reduce the volume flow required for the fore-vacuum pump. A preliminary vacuum pump is a vacuum pump designed to reduce the pressure in the pumped-out volume or pumping system from atmospheric pressure to a value at which another pumping system or vacuum pump can start operating. A single-stage vacuum pump is a vacuum pump in which the pressure difference is created by one operating stage. A multistage vacuum pump is a vacuum pump in which the pressure difference is created sequentially by several working stages (the pumping stages are numbered starting from the stage creating the highest vacuum). A mechanical vacuum pump is a gas pumping vacuum pump, the pumping action of which is based on the movement of gas due to the mechanical movement of the working parts of the pump. A positive displacement vacuum pump is a mechanical vacuum pump in which a volume filled with gas is periodically cut off from the inlet, ensuring the movement of gas to the outlet. Gaeoballast vacuum pump is a vacuum pump with an oil seal, equipped with a device for dosed supply of non-containing gas to prevent condensation of pumped vapors in the pump. A piston vacuum pump is a positive displacement vacuum pump in which compression and injection of gas occur under the action of the reciprocating movement of the piston. A rotary vacuum pump is a volumetric vacuum pump in which compression and injection of gas is carried out by rotating surfaces of a solid body. A vane rotor vacuum pump is a rotary vacuum pump in which an eccentrically mounted rotor rotates tangentially relative to the stationary surface of the stator: in this case, two or more plates sliding in the slots of the rotor and pressed against the inner stator stake divide the stator chamber into cavities with varying volumes. A vane-type vacuum pump is a rotary vacuum pump in which an eccentrically mounted rotor rotates, sliding along the inner wall of the stator; in this case, the plate, moving relative to the stator, is pressed against the rotor and divides the working chamber into parts with varying volumes. A plunger vacuum pump is a rotary vacuum pump in which an eccentrically mounted rotor rotates relative to the inner wall of the stator; in this case, a plate rigidly fixed to the rotor divides the working chamber into cavities with varying volumes and slides in a spool (plunger) oscillating in the corresponding stator socket. A liquid ring vacuum pump is a rotary vacuum pump in which an eccentrically mounted rotor with blades attached to it throws liquid towards the stator wall; the liquid takes the form of a ring located relative to the stator, and together with the rotor blades forms cavities with varying volumes. A two-rotor vacuum pump (Roots pump) is a rotary vacuum pump, the working chamber in which two mutually connected rotors, shaped like a figure of eight, synchronously rotate in opposite directions with a very small gap, without touching each other and the chamber walls. A trochoid vacuum pump is a rotational vacuum JASOS, in which the center of gravity of the ellipmant of the rotor describes the circle, and the pumping chamber of the pump has a trosiyei cross section. The kinetic vacuum pump is a mechanical vacuum OS, in which the pulse of the movement is transmitted to the gas molecules in such a way that the gas is continuously moved from the entrance to the output of the pump (distinguish the jet pumps in which the pumping occurs due to the capture of the ILN molecules with a stream of the working body, and the rotational pumps, In which the impulse of movement is transmitted to the gas molecules by moving surfaces of us for the Wasp). Vacuum turbine -kiytichv: a vacuum JASOS, in which the pulse of movement is transmitted to gas from rotating hard surfaces. The molecular vacuum pump is a kinetic vacuum pump, in which the gas molecules in the result of their contact with the surface of the high -speed rotor are reported by an impulse of movement, which makes them move towards the output of the pump. A turbochargic vacuum pump is a molecular vacuum pump, on the shaft of the rotor of which discs with slots or blades are fixed, which rotate between the corresponding stator disks. The axial vacuum turbine is a vacuum turbine, in which the movement impulse is transmitted to gas along the rotation axis. The centrifugal vacuum turbine is a vacuum turbine, in which the movement impulse is transmitted to gas in a radial direction. Inkjet vacuum pump - a gas -freeing vacuum pump, in which pumping occurs by capturing the gas with a stream of the working body (liquid, steam or gas). The ekector vacuum pump is a pair of an amulet pump, in which a turbulent -backed capture of the gas occurs with a stream. The liquid -structural vacuum pump is a jet vacuum pump, in which a stream of liquid (usually water) is used as a working body. A gas -industrial vacuum pump is a jet vacuum pump, in which a stream of gas is used as a working body. A steam -guts vacuum pump is a jet vacuum pump, in which a stream of steam is used as a working carcass. The diffusion vacuum pump is a steam -cutting high -ak pump, in which the capture of the gas occurs due to the diffusion of gas into the stream. A self -cleaning diffusion vacuum pump is a diffusion vacuum pump in which the flying impurities do not return to the boiler, but go to the exit. The fractional diffusion vacuum pump is a multi -stage vacuum steam oil pump, from the step of the lowest pressure of which the gas is pumped out with denser components of the working substance, which are a stream of pair of low pressure, and from the steps of higher pressure - less dense components with higher pair pressure. The diffusion -zeal vacuum pump is a steam -gap vacuum pump in which the steps or steps that have the characteristics of the ejector vacuum pump precede the steps or steps that have the characteristics of the diffusion vacuum iasos. The ion vacuum pump is a kinetic vacuum pump in which the gas molecules are ionized, and then move to the yasos output using an electric and magnetic fields (only an electric field). Sorption vacuum pump is a gas -based vacuum pump, in which pumping occurs due to gas sorption. The adsorption vacuum pump is a sorption vacuum JASOS, in which pumping occurs due to physical gas sorption with a porous sorbent at low temperature. A gutter vacuum pump is a sorption vacuum JASOS, in which pumping occurs mainly due to the gas -springs of gas by ghetter. The sublimation vacuum pump is a gutter vacuum JASOS, the absorbing surface of which is updated with the condensation of the IA of it the thermally evaporated gutter. Getter Noeonic vacuum pump is a gutter vacuum IASOS, in which, along with chemosorption, the gas is on the gas with the subsequent introduction of accelerated IOI into the surface of the sprayed gutter. The evaporative venue pump is a hetterioi vacuum JASOS, in which the noiziroviai gas is directed to the surface of the Getter obtained as a result of continuous or periodic evaporation. Magnetic zlestraera -rhiomal vacuum pump is a hetterioi -ia vacuum JASOS, in which a gas discharge in a magnetic field is used to spray the gutter. Vacuum curonasos - condensation or sorption pump with working surfaces cooled to ultra -low temperatures. The speed of pumping the vacuum pump is the volume of gas at fixed pressure, pumped out to a unit of time. The speed of the vacuum pump is a value characterized by the speed of pumping in the input section of the pump during its operation. Effective speed of pumping the vacuum pump is the speed of pumping at the end of the pipeline connected to the pumped vessel. The performance of the vacuum pump is the gas stream through the input cross section of the pump. The largest pressure on the vacuum pump is the highest pressure in the input cross section of the vacuum pump, in which the pump may begin to work. The largest exhaust pressure of the vacuum pump is the highest pressure in the output section of the vacuum JASOS, in which the JASOS can pump. The largest operating pressure of the vacuum pump is the highest pressure in the input cross section of the pump, in which it retains the nominal speed of the action for a long time. The maximum residual pressure of the pump is the value to which the pressure in the standardized test volume without gas production with a normally working pump is asymptotically. The time of the vacuum pump to the operating mode is the time from the moment the pump is turned on until the moment when oh can start pumping out at the operating pressure. Means for measuring and monitoring the vacuum manometer - a device for measuring pressure or a difference in pressure. Vacuum meter - a pressure gauge for measuring the pressure of a rarefied gas or steam. The absolute vacuummeter is a vacuummeter, the sensitivity of which is the same for all gases and can be calculated by the measured physical quantities. Differential vacuummeter is a vacuummeter for measuring the pressure difference on both sides of the dividing sensitive element. A vacuum meter of full pressure is a vacuummeter for measuring the total pressure provided by all components of the gas mixture. The measuring pressure converter is the primary measuring transducer that perceives directly measured pressure and converts it into another physical value. An open pressure converter is a pressure converter whose electrode system does not have a sealed case and (or) conductivity between the center of the electrode system and the input cross section of the connecting pipe is or exceeds 210 ~ 2 m3/s. A closed pressure converter is a pressure converter whose electrode system is enclosed in the sealed case and conductivity between its center and the output cross section of an annex pipe less than 2 · 10-2 m3/s. The vacuum meter measuring block is a part of the vacuum meter, which is designed to develop a measuring information signal in a form available for direct perception by the observer, and contains the power supply and all the electrical circuits necessary for the operation of the device. The deductible device of the vacuummeter is part of the measuring block of the vacuum meter designed to register the values of the measured parameter. The mass spectrometer is a device for a quantitative and (or) qualitative determination of the composition and structure of substances, the study of physicochemical processes and phenomena according to the mass spokes for these substances. An indicator with a discharge tube is a transparent gas discharge tube that allows the color of the gas and its pressure by the color and shape of the discharge. The liquid vacuummeter is a vacuum meter of full pressure, the action of which is based on balancing the measured pressure or the pressure of the pressure of the fluid column. Mobraznaya vacuummeter is a liquid vacuummeter consisting of communicating vessels, the pressure of which is determined by one NLN to several fluid levels. The deformation vacuummeter is a vacuum meter of full pressure, the action of which is based on the dependence of the deformation of the sensitive element or the strength developed by it on the measured pressure. A membrane vacuummeter is a deformation vacuummeter, in which a membrane iln membrane box is a sensitive element. The compression vacuummeter is a liquid vacuummeter, in which the latter is pre -compressed to measure the pressure of the sparse gas. A viscous vacuummeter is a vacuum of full pressure, the action of which is based on the dependence of the viscosity of the sparse gas on the speed of movement in it of the solid and measured pressure. The thermal vacuummeter is a vacuum of full, pressure, the action of which is based on the dependence of the thermal conductivity of the sparse gas on pressure. Thermoparous vacuummeter is a thermal vacuum, which uses the dependence of the electric motor thermocouple on the measured pressure. The resistance vacuum is a thermal vacuum, the action of which is based on the dependence of the electrical resistance of the element heated by the current on the gas pressure. The thermal molecular vacuummeter is a vacuum meter of full pressure, the action of which is based on the transfer to the sensitive element of the total pulse of gas molecules, reflected from surfaces with various temperatures. The ionization vacuummeter is a full pressure vacuum, the action of which is based on the dependence of the ion current that arose in the gas as a result of ionization of the molecules of rarefied gas from pressure. Radioisotopic ionization vacuummeter is an ionization vacuum, in which radioactive sources are used for gas ionization. A magnetic electrical discharge vacuum - an ionization vacuum, the action of which is based on the dependence of the tone of the electric discharge in the magnetic field on the measured pressure. Penning vacuum - a magnetic electrical discharge vacuum, in the converter of which one of the electrodes consists of two interconnected plates, and the other (usually the anode) is placed between them and has the shape of a closed frame; In this case, the direction of the magnetic field perpendicular to the plane of the anode frame. An electronic ionization vacuummeter is an ionization vacuum, in which the gas is ionized by electrons accelerated by an electric field. An electronic ionization vacuummeter with an axial manifold is an electronic ionization vacuummeter with reduced background pressure due to the use of thin wire ions as a collector, placed as a cylindrical mesh and a cathode grid. An extractor vacuummeter is an electronic ionization vacuum, in the converter of which the background current is reduced by the use of a short and hound of wire, located located in the AIOD axis and derived from the ionization. An electronic ionization vacuummeter with a magnetic field is an electronic ionization vacuum, a pressure converter of which is a cylindrical magnetron, in which the electron trajectory and the number of IOIs formed are increased under the influence of the magnetic field. Radio frequency mass spectrometer is a mass spectrometer in which IOIs are divided in a radio frequency longitudinal electric field formed by sequentially located mesh electrodes (radio frequency st cascades); wherein
Application
Light bulbs use low vacuum.
The flask is filled with gas, usually argon, at low pressure, which protects the tungsten filament. Vacuum is useful for many processes and is used in a variety of devices. For the first time for mass-used goods, it was used in incandescent lamps to protect the filament from chemical decomposition. The chemical inertness of materials provided by vacuum is also useful for electron beam welding, cold welding, vacuum packaging and vacuum frying. Ultra-high vacuum is used in the study of atomically pure substrates, since only a very high vacuum keeps surfaces clean at the atomic level for quite a long time (from minutes to days). High and ultra-high vacuums eliminate air resistance, allowing particle beams to deposit or remove materials without contamination. This principle underlies chemical vapor deposition, vacuum deposition and dry etching, which are used in the production of semiconductors and optical coatings, as well as in surface chemistry. Reducing convection provides thermal insulation in thermoses. High vacuum lowers the boiling point of liquid and promotes low temperature degassing, which is used in freeze drying, glue preparation, distillation, metallurgy and vacuum cleaning. The electrical properties of vacuum make electron microscopes and vacuum tubes, including cathode ray tubes, possible. Vacuum circuit breakers are used in electrical switchgear. Vacuum breakdown is of industrial importance for the production of certain grades of steel or high purity materials. Eliminating air friction is beneficial for flywheel and ultracentrifuge energy storage.
Vacuum driven machines
Vacuum is commonly used to produce suction, which has an even wider range of applications. Newcomen's steam engine used vacuum instead of pressure to drive the piston. In the 19th century, vacuum was used for traction on Isambard Brunel's experimental pneumatic railway. Vacuum brakes were once widely used on trains in the UK, but except on heritage railways they have been replaced by air brakes.
This shallow well pump reduces the atmospheric pressure inside its own chamber. The atmospheric vacuum expands down into the well and forces water to flow up the pipe into the pump to equalize the reduced pressure. Pumps with a ground chamber are effective only to a depth of about 9 meters, due to the weight of the water column equalizing atmospheric pressure.
Intake manifold vacuum can be used to control auxiliary equipment on vehicles. The best known application is as a vacuum booster to increase brake power. Vacuum was previously used in Autovac windshield wiper vacuum drives and fuel pumps. Some aircraft instruments (the attitude indicator and heading indicator) are usually operated by vacuum, as insurance against failure of all (electrical) instruments, since early aircraft often did not have electrical systems, and since there are two readily accessible sources of vacuum on a moving aircraft, the engine and the venturi. Vacuum induction melting uses electromagnetic induction in a vacuum.
Maintaining a vacuum in the condenser is important for the efficient operation of steam turbines. For this, a steam injector or a liquid ring pump is used. The normal vacuum maintained in the vapor volume of the condenser at the turbine exhaust (also called turbine condenser pressure) is in the range of 5 to 15 kPa, depending on the type of condenser and environmental conditions.
Degassing
Evaporation and sublimation in a vacuum is called degassing. All materials, solid or liquid, vaporize slightly (gassing occurs), and their degassing is necessary when the vacuum pressure drops below their vapor pressure. Floating materials in a vacuum has the same effect as leakage and can limit the achievable vacuum. Evaporation products can condense on nearby cooler surfaces, which can cause problems if they coat optical instruments or react with other materials. This causes great difficulties when flying in space, where a darkened telescope or solar cell could derail a high-cost operation.
The most common waste product in vacuum systems is water absorbed by the chamber materials. Its amount can be reduced by drying or heating the chamber and removing absorbent materials. Evaporating water can condense in the oil of rotary vane pumps and dramatically reduce their operating speed if a gas ballast device is not used. High vacuum systems must be kept clean and free of organic matter to minimize outgassing.
Ultra-high vacuum systems are typically annealed, preferably under vacuum, to temporarily increase the evaporation of all materials and evaporate them. Once most of the vaporized materials have been evaporated and removed, the system can be cooled to reduce vaporization of materials and minimize residual gas emissions during operational operation. Some systems are cooled significantly below room temperature using liquid nitrogen to completely stop residual gas evolution and at the same time create the effect of cryogenic pumping of the system.
Pumping and atmospheric pressure
Gases cannot be pushed out at all, so a vacuum cannot be created by suction. Suction can spread and dilute the vacuum, allowing high pressure to introduce gases into it, but the vacuum must be created before suction can occur. The easiest way to create an artificial vacuum is to expand the volume of the chamber. For example, the diaphragm muscle expands the chest cavity, which leads to an increase in lung capacity. This expansion reduces the pressure and creates a low vacuum, which is soon filled with air forced by atmospheric pressure.
To continue emptying the chamber indefinitely, without constantly using its expansion, its vacuum compartment can be closed, purged, expanded again, and so on many times. This is the operating principle of positive displacement (gas transfer) pumps, such as a manual water pump. Inside the pump, a mechanism expands a small sealed cavity to create a vacuum. Due to the pressure difference, some of the liquid from the chamber (or well, in our example) is pushed into the small cavity of the pump. The pump cavity is then sealed against the chamber, opened to the atmosphere and compressed to its minimum size, expelling the liquid.
The above explanation is a simple introduction to evacuation and is not representative of the range of pumps used. Many variations of positive displacement pumps have been developed, and many pump designs are based on radically different principles. Pulse transfer pumps, which have some similarities to dynamic pumps used at higher pressures, can provide a much higher vacuum quality than positive displacement pumps. Gas bonding pumps, capable of capturing gases in a solid or absorbed state, often operate without moving parts, without seals and without vibration. None of these pumps are universal; each type has serious application limitations. All have difficulty pumping out low molecular mass gases, especially hydrogen, helium and neon.
The lowest pressure that can be achieved in the system, in addition to the design of the pumps, also depends on many factors. Several pumps can be connected in series, in so-called stages, to achieve a higher vacuum. The choice of seals, chamber geometry, materials and pumping procedures will all have an effect. Collectively, all this is called vacuum technology. And sometimes, the resulting pressure is not the only significant characteristic. Pumping systems are characterized by oil contamination, vibration, selective pumping of certain gases, pumping speeds, intermittent operation, reliability or resistance to high leak rates.
In ultra-high vacuum systems there are some very strange leak paths and vapor sources to consider. The water absorption capacity of aluminum and palladium becomes an unacceptable source of evaporation; even the adsorption capacity of hard metals such as stainless steel or titanium must be taken into account. Some oils and greases will boil under high vacuum. It may be necessary to take into account the permeability of the metal walls of the chambers, and the direction of the grains of the metal flanges should be parallel to the end of the flange.
The lowest pressures currently achievable in laboratory conditions are about 10-13 torr (13 pPa). However, pressures lower than 5×10-17 torr (6.7 fPa) have been indirectly measured by a cryogenic vacuum system. This corresponds to ≈100 particles/cm3.
Some examples of sous vide cooking
Chicken fillet
Working with chicken using sous-vide technology probably gave the most outstanding results. Firstly, in terms of mass loss; secondly, in terms of taste.
We boiled four chicken breast samples, marinated in BBQ sauce with a mixture of three peppers and garlic powder on the first day of the experiment and left alone on the second day. We cooked chicken in the same way.
The sample weights (day two) were: 136 g, 140 g, 137 g and 119 g, respectively.
We cooked the chicken for 1 hour 9 minutes to 1 hour 25 minutes at 60.5°C.
The thickness of the pieces was approximately 25 mm.
This experiment was required in order to obtain information about weight loss during the longest sous-vide processing to achieve well-done cooking.
The weight of the finished product was, respectively, 120 g, 125 g, 124 g and 111 g (11.7%, 10.7%, 9.4%, 6.7%). It is significant that with increasing cooking temperature the weight loss was less. This is explained by the fact that during longer processing the process of lipid gelation accelerates, that is, the gelatinization of moisture in the product increases.
Classic temperatures and cooking times depending on the thickness of the pieces are presented in Table 2.
Table 2 - Classic temperatures and cooking times depending on the thickness of the pieces
The mushrooms were cooked a little differently. We cooked fresh mushrooms at 65.5°C for 50 minutes and achieved a weight loss of only 6%.
At the end of the experiment we used mushrooms, brisket and bell peppers cooked al dente to make pizza.
King prawns
The king prawns didn't give us much hope of achieving a stellar sous-vide result. We worked with frozen black tiger shrimp at 60.5°C for 10, 12, 15, 17 and 20 minutes. The weight loss ranged from 29–33%. The shrimp, just like the chicken, ended up on the pizza after sous-vide, which we happily ate after the experiment.
The shrimp were much more tender than usual, and as they were cooked and quickly cooled on ice, they developed bright red streaks, much more vibrant than the normal treatment. The mass loss, unfortunately, turned out to be far from being as small as we expected. We concluded that other than minor flavor changes, the product did not perform particularly well.
Meat loaf
We have always been curious to work with the cheapest products. This time we chose home-made ready-made minced pork and beef, which we purchased at the Metro Cash & Carry supermarket. We added salt, pepper and a loaf of bread soaked in milk to the minced meat. Then beat the mass in a planetary mixer.
After preparing the mass, we placed it in a polyethylene terephthalate plastic tray and placed the tray in a vacuum bag. During the vacuuming process, the air entering the tray almost doubled the volume of the mass and it was squeezed out of the bag. We had to re-fill the bag with new cutlet mass. This time we made an investment exactly 50% less than the first time. This time everything went fine. The volume of the mass doubled and then returned to its previous size when the air suction cycle was activated. We placed the tray in a bag in a gastronorm container with a thermal circulator at a temperature of 67.5 °C. The temperature was so high because the mince was clearly dominated by pork, which requires a much higher processing temperature than beef.
The product was cooked for 1 hour 25 minutes. Then we took out the packaging, removed the bag and cut the loaf of bread into 20mm thick pieces, placing them on a frying sheet. The top of the bread was glazed with a ready-made mixture of Santa Maria pineapple sauce. Then the product was baked in a combi oven at a temperature of 200 °C for 12 minutes. The weight loss was 12% after cooking and another 3.5% after convection.
Turkey shawarma
Shawarma, shawarma, shawarma, shuarma, shaorma, in some countries called dener kebab, donar (from Turkish döner kebab) is a Middle Eastern dish (probably of Turkish origin) made from pita bread or pita bread stuffed with minced fried meat (lamb, chicken, pork , less often veal, turkey) with the addition of spices, sauces and fresh vegetable salad.
Shawarma - originally the name of a Turkmen dish invented by steppe shepherds - the boiled meat of a gazelle or saiga is finely chopped and placed in the washed stomach of the same gazelle or saiga, and its fat is poured into it. Then the stomach is sutured. It can be stored for up to several months without spoiling.
We prepared Israeli turkey shawarma. We cooked turkey fillet pieces in sous-vide for 1 hour at a temperature of 58 °C.
Then we prepared an Arabic salad (tomatoes, cucumbers, lemon, black pepper), fried French fries, chopped pickles and pickled peppers, prepared tahini (an Arabic sauce made from sesame seeds and olive oil), pan-fried the turkey, wrapped the ingredients in pita type lafa (Armenian lavash) and placed in a convection oven for 5 minutes at a temperature of 200 °C.
Salmon
The purpose of this experiment is to select the optimal temperature for heat treatment of salmon using a sous-vide thermal circulator to achieve the best taste of the product, its texture and color. As part of this experiment, we will also identify heat treatment extremes, at the occurrence of which the product loses its attractiveness. The experiment uses salmon half steaks weighing 90 grams after removing the skin and removing the bones. The salmon is washed and placed in a 10% salt solution in water at a temperature of about 15 °C to avoid intense protein coagulation during processing.
The product is then placed in a vacuum bag measuring 20 x 30 cm. It is at this point that salt, spices and herbs are added to the product. (We did not use additives in this experiment.)
We recommend vacuum sealing one piece of product in one bag if the serving will be textured (when the meat is served in one piece, its attractive appearance is especially important). In the case of non-textured serving (for example, adding salmon to a salad and then stirring), you can add 2-3 parts of the product in order to save money on bags.
Important!
Pour hot water from the kettle into the sous-vide container before turning on the circulator.
Do not place the product in the bath before the circulator signals that it has reached the set temperature.
It is recommended to place the vacuum-sealed product overnight in a medium-temperature (in the food industry, medium-temperature is the standard from 4 °C to 6 °C) refrigerator for additional fermentation of the product.
It is recommended to vacuum the product for no more than 40–45 seconds. Or when the vacuum is set to 50% (depending on the modification of the vacuum apparatus).
In this experiment, a vacuum apparatus of the Dutch “Jambo mini” model was used.
Salmon is a very soft and cavernous product, so it should be processed in a biokinetic temperature range of 35 to 50 °C for no more than 20-25 minutes.
Below is a table of 5 recommended temperatures for processing salmon, developed by (Johannesburg) - the largest supplier of fish and seafood in Africa.
Table 3 – Recommended processing temperatures for salmon
It is important to note that cooking salmon and any other type of salmon in sous vide often continues in the form of additional cooking on the grill or frying pan to color the product. Combined cooking of red fish is practiced by most sous-vide school leaders in the world.
Our experiment differed somewhat in both cooking time and temperature conditions from African specialists.
We worked according to Douglas Baldwin's table (Table 6), which is given below, and obtained equally impressive results.
Table 4 – Time and temperatures for low-temperature processing of salmon fish
Pork
Working with pork, we were primarily interested in the dorso-lumbar (cutlet) part, which, as is known, consists of parts of the neck cut, loin and sirloin. It is usually cut off from the chest, starting at the fourth or fifth rib. Sirloin cutlets are especially tender and lean, and have a low bone yield. The loin is used to produce cutlets on the bone and intercostal cutlets. If cutlets are chopped and graded for serving as fixed-weight portions, varying bone yields must be taken into account. Many suppliers bring in the carcass parts used for chops, cut them into slices and offer them to restaurants as schnitzels or pork steaks. Since the area of the slices is small, they are cut into “butterfly steak”. The neck part has layers of fat and is therefore well suited for stewing. It can also be cut into chops from the neck part. Thin slices from the boneless neck are suitable for cordon bleu-type dishes. Slices of boiled smoked ham and Swiss cheese are placed between the slices of meat and secured with small skewers.
We chose gas packed neck steaks and cooked them at 85°C for 1 hour 25 minutes. The steaks were then cooked in a convection oven at 200 °C for 9 minutes.
"The Perfect Egg"
Cooking the so-called “perfect egg” involves special methods of working with the texture of the product and its morphology. At a temperature of 64.5 °C, conalbumin in the egg proteins is denatured, as a result of which both the yolk and the white turn into a viscous adhesive mass. McGee tests eggs for doneness at various temperatures ranging from 57.8 to 66.7°C in 1.1°C intervals. We use different tactics, achieving the same result. The results of our work seemed very encouraging to us and promising for use both in confectionery products and in salads and hot dishes. We obtained amazing results when processing the product at a temperature of 67.5 °C.
Notches were made at 45, 50, 55, 60, 65 and 75 minutes.
The experiment showed that the effect of low temperature on raw chicken eggs allows:
- change and vary the consistency of the yolk and white;
- encapsulate the yolk and give it a perfect round shape and glossy appearance;
- change the saturation of yolk pigmentation;
- ensure easy separation of the egg contents from the shell;
- pasteurize the egg at a temperature of 64.5 °C for 75 minutes and store the egg without changes in physicochemical and microbiological parameters for up to 14 days.
Vegetable stew with meat
Note that low-temperature cooking of vegetables allows you to take advantage of all the main advantages of this technology.
These include:
- minimal weight loss of vegetables;
- preservation of nutrient composition (minimal loss of vitamins A, B and C);
- delicate taste and aroma due to cooking in its own juice;
- minimal consumption of spices (40% less than with conventional cooking);
- no denaturation of the product and no loss of color.
We decided to work with potatoes, carrots, bell peppers and mushrooms. We had leftover sous-vide chicken breast from a previous experiment, and we also decided to add it to our vegetable and mushroom stew.
Bell peppers, potatoes and carrots were vacuum-sealed separately. Then we vacuum-sealed the potatoes and carrots in one bag and got an interesting result: the new potatoes, which certainly cooked 3 times faster than the carrots, completely absorbed the carrot juice, and the vegetables came out with virtually no moisture. We processed the products at a temperature of 85 °C for 1 hour 40 minutes.
The bell peppers were cooked at the same temperature for 35 minutes.
The peppers and carrots turned out al dente. The vegetables were then fried over high heat with minimal additions of vegetable oil, pepper and salt in a frying pan and stewed in a saucepan with butter added at the end.
Chicken hearts
Chicken hearts are often used to prepare oriental dishes. As a rule, this by-product is sold frozen. Chilled chicken hearts are quite rare, even in poultry farm stores; this is due to the peculiarities of poultry slaughter technology. If you come across chilled chicken hearts, then most likely the bird was gutted in the culinary department at the store or the heart was previously defrosted and placed on the counter. There are many recipes for making chicken hearts. These include: hearts in beer, in sour cream, in kefir, in teriyaki sauce, in cheese sauce, with apples, with mushrooms, etc. The main problem when preparing hearts is softening of the product. As a rule, the hearts are pre-marinated or soaked in a 10% salt solution for 1 to 5 hours. Soy marinade helps soften the product well. Some chefs use special softeners.
The purpose of the experiment was to prepare chicken hearts in a sauce made from a mixture of sesame, olive oil and soy sauce and without it.
Raw materials: domestically produced frozen chicken hearts. Weight before defrosting – 890 grams, weight after defrosting – 720 grams.
Processing time: 2 hours 15 minutes.
Processing temperature: 58.5 °C.
Tools: ICC thermal circulator, frying pan.
Weight loss when cooking in sous-vide is 17%.
The loss in weight of chicken hearts was the most significant among all products participating in the experiment.
Notes
- Chambers, Austin.
Modern Vacuum Physics. - Boca Raton: CRC Press, 2004. - ISBN 0-8493-2438-6. - Tadokoro, M. (1968). "A Study of the Local Group by Use of the Virial Theorem". Publications of the Astronomical Society of Japan 20
. Bibcode: 1968PASJ…20..230T. - Rodin A. M., Druzhinin A. V.
Vacuum // Physical encyclopedia: [in 5 volumes] / Ch. ed. A. M. Prokhorov. - M.: Soviet Encyclopedia, 1988. - T. 1: Aaronov - Bohm effect - Long lines. — P. 235-236. — 707 p. — 100,000 copies. - Werner S. Weiglhofer.
§ 4.1 The classical vacuum as reference medium // Introduction to complex media for optics and electromagnetics / Werner S. Weiglhofer and Akhlesh Lakhtakia, eds. - SPIE Press, 2003. - P. 28, 34. - ISBN 978-0-8194-4947-4. - Tom G. MacKay.
Electromagnetic Fields in Linear Bianisotropic Mediums // Progress in Optics, Volume 51 / Emil Wolf. - Elsevier, 2008. - P. 143. - ISBN 978-0-444-52038-8. - Physical encyclopedia, vol.5. Stroboscopic devices - Brightness/ Ch. ed. A. M. Prokhorov. Editorial team: A. M. Baldin, A. M. Bonch-Bruevich and others - M.: Great Russian Encyclopedia, 1994, 1998.-760 pp.: ill. ISBN 5-85270-101-7, p.644
- Galileo G.
Selected works in two volumes. / Compiled by W. I. Frankfurt. - Volume 2. - M.: Nauka, 1964. - Schotti HG Technica Curiosa. 1664.
- Horror Vacui? - Raffaello Magiotti (1597-1656) - IMSS.
- Cornelis De Waard. L'experience barometrique. Ses antecedents et ses explications. Thouars, 1936. P. 181.
- How to Make an Experimental Geissler Tube
, Popular Science monthly, February 1919, Unnumbered page, Scanned by Google Books: https://books.google.com/books?id=7igDAAAAMBAJ&pg=PT3 - V. P. Borisov (Institute of the History of Natural Science and Technology named after S. I. Vavilov RAS)
. The invention that paved the way for discoveries: 2002 marked the 400th anniversary of the birth of the inventor of the vacuum pump, Otto von Guericke // Bulletin of the Russian Academy of Sciences. - 2003. - T. 73, No. 8. - P. 744-748. - V. P. Borisov, The invention of the vacuum pump and the collapse of the dogma “Fear of the Void” // Questions of the history of natural science and technology, No. 4, 2002
- Landis, Geoffrey
Human Exposure to Vacuum. www.geoffreylandis.com (7 August 2007). Retrieved March 25, 2006. - Billings, Charles E.
Chapter 1) Barometric Pressure // Bioastronautics Data Book / Parker, James F.; West, Vita R.. - Second. - NASA, 1973. - P. 5. - ISBN NASA SP-3006. - Webb P. (1968). "The Space Activity Suit: An Elastic Leotard for Extravehicular Activity." Aerospace Medicine 39
(4):376–383. PMID 4872696. - Cooke JP, RW Bancroft (1966). "Some Cardiovascular Responses in Anesthetized Dogs During Repeated Decompressions to a Near-Vacuum". Aerospace Medicine 37
(11):1148–1152. PMID 5972265. - Harding, Richard M.
Survival in Space: Medical Problems of Manned Spaceflight. - Routledge, 1989. - ISBN 0-415-00253-2.. - American Vacuum Society.
Glossary.
AVS Reference Guide
. Retrieved March 15, 2006. Archived June 15, 2013. - National Physical Laboratory, UK.
What do 'high vacuum' and 'low vacuum' mean? (FAQ - Pressure). Retrieved April 22, 2012. Archived June 15, 2013.