According to the number of structural links
- Monosaccharides - contain one structural unit.
- Oligosaccharides - contain from 2 to 10 structural units (disaccharides, trisaccharides, etc.).
- Polysaccharides - contain n structural units.
Some essential carbohydrates:
| Monosaccharides | Disaccharides | Polysaccharides |
| Glucose C6H12O6 Fructose C6H12O6 Ribose C5H10O5 Deoxyribose C5H10O4 | Sucrose С12Н22О11 Lactose С12Н22О11 Maltose С12Н22О11 Cellobiose С12Н22О11 | Cellulose (C6H10O5)n Starch (C6H10O5)n |
Groups of carbohydrates. Breakdown of carbohydrates in the body
Based on the speed at which the body processes saccharides, they are divided into 2 groups: simple (fast) and complex (slow).
Simple, or fast, carbohydrates
Fast, easily digestible ones include glucides consisting of 1 or 2 molecules, for example, glucose, sucrose. Such substances are quickly processed in the body, since their simple composition facilitates their absorption.
It is believed that products containing simple saccharides have a bad effect on a slim figure. This happens because blood glucose levels rise quickly. The body strives to normalize the amount of sugar. As a result, it is stored as fat reserves.
In addition to the detrimental effect on your figure, constant sharp jumps in glucose levels affect hormonal levels and force the pancreas to work intensively.
Complex, or slow, carbohydrates
Glucides with 3 or more molecules are called complex, or slow. It is believed that the body takes longer to process them, since simple molecules are first isolated from complex chains, and then the body assimilates them. Consumption of such glucides is more preferable than fast ones. They provide a feeling of fullness for a long time and promote good functioning of the gastrointestinal tract. Complex saccharides have a beneficial effect on the health of the digestive system because... Most of them are found in foods rich in fiber.
Reaction with concentrated sulfuric acid
Concentrated sulfuric acid removes water from carbohydrates, producing carbon C (“char”) and water.
| For example, when concentrated sulfuric acid acts on glucose, carbon and water are formed |
C6H12O6 → 6C + 6H2O
| Monosaccharides are heterofunctional compounds; their molecules contain one carbonyl group (aldehyde or ketone group) and several hydroxyl groups. |
Monosaccharides are structural units of oligosaccharides and polysaccharides.
The most important monosaccharides
| Name and formula | Glucose C6H12O6 | Fructose C6H12O6 | Ribose C6H12O6 |
| Structural formula | |||
| Classification |
|
|
|
Glucose is an aldehyde alcohol (aldose).
It contains six carbon atoms, one aldehyde and five hydroxyl groups.
Glucose exists in solutions not only in the form of linear, but also cyclic forms (alpha and beta) , which are pyranose (contain six units):
| α-glucose | β-glucose |
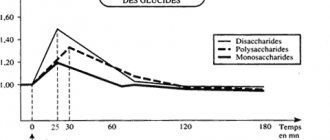
Stages of saccharide breakdown
Before considering the features of biochemical reactions in the body and the influence of carbohydrate metabolism on athletic performance, we will study the process of breakdown of saccharides with their further transformation into the very glycogen that athletes so desperately extract and spend during preparation for competitions.
Stage 1 – preliminary digestion with saliva
Unlike proteins and fats, carbohydrates begin to break down almost immediately after entering the oral cavity. The fact is that most of the products entering the body contain complex starchy carbohydrates, which, under the influence of saliva, namely the amylase enzyme included in its composition, and the mechanical factor are broken down into simple saccharides.
Stage 2 – influence of stomach acid on further breakdown
This is where stomach acid comes into play. It breaks down complex saccharides that are not exposed to saliva. In particular, under the action of enzymes, lactose is broken down into galactose, which is subsequently converted into glucose.
Stage 3 – absorption of glucose into the blood
At this stage, almost all fermented fast glucose is directly absorbed into the blood, bypassing the fermentation processes in the liver. Energy levels increase dramatically and the blood becomes more saturated.
Stage 4 – satiety and insulin response
Under the influence of glucose, the blood thickens, which makes it difficult to move and transport oxygen. Glucose replaces oxygen, which causes a protective reaction - a decrease in the amount of carbohydrates in the blood.
Insulin and glucagon enter the plasma from the pancreas.
The first opens transport cells for the movement of sugar into them, which restores the lost balance of substances. Glucagon, in turn, reduces the synthesis of glucose from glycogen (consumption of internal energy sources), and insulin “perforates” the main cells of the body and places glucose there in the form of glycogen or lipids.
Stage 5 – Carbohydrate Metabolism in the Liver
On the way to complete digestion, carbohydrates encounter the body’s main defender – liver cells. It is in these cells that carbohydrates, under the influence of special acids, are linked into the simplest chains - glycogen.
Stage 6 – Glycogen or Fat
The liver can only process a certain amount of monosaccharides in the blood. The increasing insulin level forces her to do this as soon as possible. If the liver does not have time to convert glucose into glycogen, a lipid reaction occurs: all free glucose is converted into simple fats by binding with acids. The body does this in order to leave a reserve, but due to our constant nutrition, it “forgets” to digest, and the glucose chains, turning into plastic fatty tissue, are transported under the skin.
Stage 7 – secondary cleavage
If the liver has coped with the sugar load and was able to convert all carbohydrates into glycogen, the latter, under the influence of the hormone insulin, manages to be stored in the muscles. Further, under conditions of lack of oxygen, it is broken down back to the simplest glucose, not returning to the general bloodstream, but remaining in the muscles. Thus, bypassing the liver, glycogen supplies energy for specific muscle contractions, while increasing endurance (source - Wikipedia).
This process is often called the “second wind”. When an athlete has large reserves of glycogen and simple visceral fats, they will only be converted into pure energy in the absence of oxygen. In turn, the alcohols contained in fatty acids will stimulate additional vasodilation, which will lead to better susceptibility of cells to oxygen in conditions of its deficiency.
Chemical properties of glucose
Aqueous glucose solution
In an aqueous solution of glucose, there is a dynamic equilibrium between two cyclic forms - α and β and a linear form:
Qualitative reaction to polyhydric alcohols: reaction with freshly precipitated copper (II) hydroxide
When freshly precipitated copper (II) hydroxide reacts with glucose (and other monosaccharides), the hydroxide dissolves to form a blue complex.
Reactions to carbonyl group - CH=O
Glucose exhibits properties characteristic of aldehydes.
- The “silver mirror” reaction
- Reaction with copper(II) hydroxide when heated. When glucose interacts with copper (II) hydroxide, a brick-red precipitate of copper (I) oxide precipitates:
- Oxidation with bromine water. When glucose is oxidized with bromine water, gluconic acid is formed:
- Glucose can also be oxidized with chlorine, berthollet salt, and nitric acid.
| Concentrated nitric acid oxidizes not only the aldehyde group, but also the hydroxo group at the other end of the carbon chain. |
- Catalytic hydrogenation. When glucose interacts with hydrogen, the carbonyl group is reduced to an alcohol hydroxyl, and a hexahydric alcohol , sorbitol, is formed:
- Fermentation of glucose. Fermentation is a biochemical process based on redox transformations of organic compounds under anaerobic conditions.
Alcoholic fermentation. During alcoholic fermentation of glucose, alcohol and carbon dioxide are formed:
C6H12O6 → 2C2H5OH + 2CO2
Lactic acid fermentation. During alcoholic fermentation of glucose, alcohol and carbon dioxide are formed:
Butyric acid fermentation. During alcoholic fermentation of glucose, alcohol and carbon dioxide are formed:
- Formation of glucose esters (characteristic of the cyclic form of glucose).
Glucose is capable of forming ethers and esters.
The hemiacetal (glycosidic) hydroxyl is most easily replaced.
For example, α-D-glucose reacts with methanol.
This produces glucose monomethyl ester (α-O-methyl-D-glucoside):
| Glucose ethers are called glycosides. |
Under more severe conditions (for example, with CH3-I ), alkylation is possible at other remaining hydroxyl groups .
Monosaccharides are capable of forming esters with both mineral and carboxylic acids.
| For example , β-D-glucose reacts with acetic anhydride in a 1:5 ratio to form glucose pentaacetate (β-pentaacetyl-D-glucose): |
What carbohydrates cleanse the body?
Our intestines must not only absorb nutrients from food well, but also regularly remove toxins. And carbohydrates help him with this.
As already mentioned, our body is equipped with various effective self-cleaning mechanisms. The main routes of elimination of harmful substances are through the lungs, liver, intestines (with stool), skin (with sweat) and kidneys (with urine).
However, for most people, the organs responsible for cleansing are constantly working in an intense mode and are under serious stress. For example, the liver, which is rightly called the main filter of the body, often has to deal with fatty foods, chemical food additives, alcohol, and a large number of medications. Constipation aggravates the process - the intestines do not remove waste, but instead absorb and accumulate toxins, which leads to self-poisoning of the body. To prevent this from happening, you should definitely include in your diet a sufficient amount of dietary fiber, which will restore motility and ensure daily bowel movements. These include fiber (cellulose), which forms the membranes of plant cells, and pectins.
On average, the daily carbohydrate requirement of healthy people is 400 g for men and 350 g for women . With dietary nutrition, especially against the background of obesity, the carbohydrate content in food can be reduced to 200 g. The physiological minimum, below which one should not fall, is about 100 g of carbohydrates per day.
Connecting these cells to each other. Fiber stimulates intestinal motor function and bile secretion, forms feces, creates a feeling of fullness, and helps remove cholesterol from the body. Pectins absorb harmful substances, reduce putrefactive processes in the intestines, and promote the healing of its mucous membrane. These properties of pectins are widely used in the treatment of diseases of the gastrointestinal tract. Fruits, berries and some vegetables (beets, carrots, parsley) are rich in pectins.
A long-term lack of “ballast” substances in the diet leads to constipation, contributes to the occurrence of diverticula, polyposis, hemorrhoids and even colon and rectal cancer, and is also one of the risk factors in the development of atherosclerosis, diabetes mellitus, and cholelithiasis. However, excessive consumption of dietary fiber is also harmful, since it leads to bloating, increased gas formation with flatulence, and deterioration in the absorption of proteins, fats, calcium, iron and other microelements.
Fiber content in various foods
Below is the amount of fiber (g) per 100 g of product.
- Very high (more than 1.5) - Wheat bran, beans, nuts, oatmeal, fresh mushrooms, raspberries, strawberries, blueberries, black, white and red currants, cranberries, gooseberries, prunes.
- High (1–1.5) - Buckwheat, pearl barley and barley, Hercules oat flakes, split peas, potatoes, carrots, white cabbage, green peas, eggplants, sweet bell peppers, pumpkin, sorrel, quince, oranges, lemons , cowberry.
- Moderate (0.6–0.9) - Rye bread, millet, green onions, cucumbers, beets, tomatoes, radishes, cauliflower, melon, apricots, pears, peaches, apples, bananas, tangerines.
- Low (0.3–0.5) - Rice, zucchini, lettuce, watermelon, cherries, plums, cherries.
Products enriched with fiber: rye bran is very rich in B vitamins, magnesium, potassium, and fiber. They are added to flour products, cereals, soups, compotes, and are also used to prepare a healing drink - bran decoction. Bran is included in diets for hypertension, diabetes, atherosclerosis, obesity, constipation, and cholelithiasis. Particularly useful are non-carbohydrate bran washed from starch.
Protein-bran bread with an increase in protein content and a decrease in the amount of carbohydrates (up to 23% and 16%, respectively) is a dietary product. This bread contains a lot of B vitamins, microelements and fiber. But at the same time, its energy value is only 0.76 MJ (180 kcal). Protein-bran bread is included in diets for diabetes, obesity and other diseases associated with metabolic disorders.
Doctor's bread with bran is also characterized by a high content of B vitamins, microelements and fiber. Included in diets for constipation, atherosclerosis, coronary heart disease and cholelithiasis.
Salt-free bread made from rye flour contains only 52 mg of sodium per 100 g of product (instead of 300–400 mg in other types of bread). It is included in diets for hypertension, circulatory disorders, and kidney diseases.
Today we consume on average 33% less fiber than we did a hundred years ago.
Obtaining glucose
Starch hydrolysis
In the presence of acids, starch is hydrolyzed:
(C6H10O5)n + nH2O → nC6H12O6
Synthesis from formaldehyde
The reaction was first studied by A.M. Butlerov. The synthesis takes place in the presence of calcium hydroxide:
6CH2=On → C6H12O6
Photosynthesis
In plants, carbohydrates are formed as a result of the reaction of photosynthesis from CO2 and H2O:
6CO2 + 6H2O → C6H12O6 + 6O2
| Fructose is a structural isomer of glucose. This is a ketone alcohol (ketose): it can also exist in cyclic forms (furanose). |
It contains six carbon atoms, one ketone group and five hydroxyl groups.
| Fructose | α-D-fructose | β-D-fructose |
Fructose is a crystalline substance, highly soluble in water, sweeter than glucose.
It is found freely in honey and fruits.
The chemical properties of fructose are associated with the presence of ketone and five hydroxyl groups.
Hydrogenation of fructose also produces sorbitol.
| Disaccharides are carbohydrates whose molecules consist of two monosaccharide residues connected to each other through the interaction of hydroxyl groups (two hemiacetal or one hemiacetal and one alcohol). |
Carbohydrates (sugars) are organic substances that have a similar structure and properties, the composition of most of which is reflected by the formula Cx(H2O)y ,
where x, y ≥ 3.
Well-known representatives: glucose (grape sugar) C6H12O6, sucrose (cane, beet sugar) C12H22O11, maltose (malt sugar) C12H22O11, lactose (milk sugar) C12H22O11, starch and cellulose (C6H10O5)n.
Educational film "Carbohydrates"
Compounds related to carbohydrates are also known, the composition of which does not correspond to the general formula, for example, rhamnose sugar C6H12O5
At the same time, there are substances that correspond to the general formula of carbohydrates, but do not exhibit their properties (for example, the natural hexahydric alcohol inositol C6H12O6).
Carbohydrates comprise a variety of compounds - from low molecular weight ones, consisting of some atoms (x=3), to polymers [CxH2Oy]n with a molecular weight of several million (n=10000).
Biological role of carbohydrates
Carbohydrates are found in the cells of plant and animal organisms and, by weight, make up the bulk of organic matter on Earth. These compounds are formed by plants during photosynthesis from carbon dioxide and water and with the participation of chlorophyll.
Animal organisms are not able to synthesize carbohydrates and obtain them from plant foods. Carbohydrates make up a significant proportion of mammalian food.
Photosynthesis can be considered as a process reduction using solar energy:
During respiration, carbohydrates are oxidized, resulting in the release of energy necessary for the functioning of living organisms:
Video "The mechanism of photosynthesis"
The carbohydrate content in plants is up to 80% of the dry matter mass, in human and animal organisms – up to 20%. They play an important role in physiological processes. Human food consists of approximately 70% carbohydrates.
The functions of carbohydrates in living organisms are varied.
They serve as a source of reserve energy (in plants - starch, in animal organisms - glycogen). In plant organisms, carbohydrates are the basis of cell membranes. As one of the structural components, carbohydrate residues are part of nucleic acids.
Classification of carbohydrates
All carbohydrates, according to the number of structural units included in their molecules (residues of the simplest carbohydrates) and the ability to hydrolyze, can be divided into two groups: simple carbohydrates , or monosaccharides, and complex carbohydrates (oligosaccharides and polysaccharides).
Simple carbohydrates (monosaccharides) are the simplest carbohydrates that do not hydrolyze to form simpler carbohydrates.
Complex carbohydrates (oligosaccharides and polysaccharides) are carbohydrates whose molecules consist of two or more monosaccharide residues and are broken down into these monosaccharides during hydrolysis.
monosaccharides are divided into tetroses (C4H8O4), pentoses (C5H10O5), and hexoses (C6H12O6). The most important pentoses are ribose and deoxyribose, and hexoses are glucose and fructose.
Oligosaccharides (condensation products of two or more monosaccharide molecules). Among the oligosaccharides, the most important are disaccharides (dioses) - condensation products of two monosaccharide molecules (for example, sucrose - C12H22O11, upon hydrolysis it turns into a mixture of glucose and fructose).
Polysaccharides (starch, cellulose) are formed by a large number of monosaccharide molecules.
Oligo- and polysaccharides are broken down during hydrolysis to monosaccharides. Oligosaccharide molecules contain from 2 to 10 monosaccharide residues, while polysaccharides contain from 10 to 3000-5000.
Raffinose – found in sugar beets.
Glycogen is animal starch.
Nomenclature of carbohydrates
For most carbohydrates, trivial names are accepted with the suffix - ose (glucose, ribose, sucrose, cellulose, etc.).
Monosaccharides. Glucose
Disaccharides. Sucrose.
Polysaccharides. Starch. Cellulose
Sucrose (beet or cane sugar) С12Н22О11
The sucrose molecule consists of α-glucose and β-fructose residues connected to each other:
In the sucrose molecule, the glycosidic carbon atom of glucose is bonded due to the formation of an oxygen bridge with fructose, so sucrose does not form an open (aldehyde) form.
| Therefore, sucrose does not react with the aldehyde group with an ammonia solution of silver oxide and copper hydroxide when heated. Such disaccharides are called non-reducing, i.e. not capable of oxidation. |
Sucrose undergoes hydrolysis by acidified water. In this case, glucose and fructose are formed:
C12H22O11 + 6H2O → C6H12O6 + C6H12O6
glucose fructose
The role and importance of carbohydrates in the human body
The importance of carbohydrates lies in the fact that the energy supplied to the body with them is necessary not only for good health and a surge of vitality in a person. It ensures cell growth and division, and in some cases, proper metabolism.
The role of carbohydrates is important if you need a quick burst of strength, because... the body does not spend a lot of resources and time on digesting them, and they release energy instantly.
The benefits of carbohydrates for the body include maintaining the correct pH. Impaired carbohydrate metabolism leads to cell depletion.
In an attempt to compensate for the lack of nutrients, the body begins to break down fats, resulting in the release of elements such as ketones. Their excess can affect the acid-base balance of the body towards oxidation.
Carbohydrate metabolism
Glucides make up 75% of the daily diet. They provide half the calories you need. As a result of the digestion of carbohydrate-containing food, the body produces many organic compounds, from which lipids, amino acids, and nucleotides are subsequently synthesized. Glucoronides, which are also produced when saccharides are consumed, help cleanse the body of harmful substances coming from the environment or produced by internal organs.
Glucides can not only enter the body with food, but also be produced from amino acids, glycerol, and lactic acid. Therefore, they cannot be called irreplaceable. But the role of carbohydrates in nutrition is so important that if they do not come from outside, hypoglycemia may develop.
Carbohydrates and insulin
1 liter of blood contains 1 g of glucose. If the level drops below, the pancreas begins to produce the hormone glucagon, which increases blood sugar. When consuming any carbohydrate-containing product on an empty stomach, the amount of glucose in the blood increases to a maximum 30 minutes after eating. This condition is called hyperglycemia. At this stage, the pancreas begins to produce insulin. It causes glucose to accumulate in the liver and muscles, reducing its concentration in the blood.
The benefit of carbohydrates for the body is that during hypoglycemia they help quickly increase blood sugar.
Daily carbohydrate intake
The rate of glucide consumption differs for different categories of people depending on age and lifestyle:
- children under 1 year – 13 g per 1 kg of body weight;
- an adult man with an average level of physical activity under 30 years old - 300-350 g per day, over 30 - 250-300 g;
- a woman should consume 30-50 g less saccharides than a man;
- with increased physical activity, the norm increases by 40-50 g.
There are foods rich in saccharides, preferably in the first half of the day, in order to have time to use up the energy received, since it has been established in the cells of which human organ carbohydrates that remain unclaimed are deposited - in the liver.
Why are low-carb diets dangerous?
The effect of carbohydrates on the human body is so important that their deficiency has a detrimental effect on various organs and systems:
- From the digestive system, gastritis, ulcers, and disturbances in intestinal microflora may develop.
- The load on the liver and kidneys increases.
- Physically the person does not feel the best. He becomes weak, lethargic, constantly wants to sleep, has a headache, and digestion processes are disrupted. All this is a consequence of the body losing the minerals and vitamins it needs.
- The brain and nervous system suffer. Thought processes slow down. Irritability appears.
What are the dangers of excess or lack of carbohydrates?
Excess carbohydrates can cause metabolic disorders. This, in turn, causes the following violations:
- slow, difficult digestion of food;
- hormonal balance is disrupted;
- increasing the proportion of adipose tissue in the body;
- The pancreas is no longer able to provide the body with the required amount of insulin, resulting in diabetes mellitus.
From the circulatory system, there is a danger of thrombosis, as the number of platelets increases. The likelihood of a heart attack or stroke increases, because... the walls of blood vessels become thinner. All these phenomena are a consequence of an excess of carbohydrates.
Chronic deficiency of glucides is no less dangerous. It is accompanied by the deposition of fats in the liver and oxidation of the body.
Maltose С12Н22О11
It is a disaccharide consisting of two α-glucose residues ; it is an intermediate substance in the hydrolysis of starch.
| Maltose is a reducing disaccharide (one of the cyclic units can open into an aldehyde group) and undergoes reactions characteristic of aldehydes. |
The hydrolysis of maltose produces glucose.
C12H22O11 + H2O → 2C6H12O6
It is a disaccharide consisting of two α-glucose residues ; it is an intermediate substance in the hydrolysis of starch.
| Polysaccharides are natural high-molecular carbohydrates, the macromolecules of which consist of monosaccharide residues. |
The main representatives - starch and cellulose - are built from the remains of one monosaccharide - glucose.
Starch and cellulose have the same molecular formula: (C6H10O5)n , but completely different properties.
This is explained by the peculiarities of their spatial structure.
Starch consists of α-glucose residues, and cellulose - from β-glucose, which are spatial isomers and differ only in the position of one hydroxyl group:
What are the dangers of excess carbohydrates for the body?
A large amount of carbohydrates in food leads to metabolic disorders and diseases . With a balanced diet, up to 30% of food carbohydrates can turn into fats. With an excess of carbohydrates, this percentage increases significantly, which inevitably leads to the accumulation of excess weight. Therefore, when treating obesity, it is important to limit the consumption of easily digestible carbohydrates.
Systematic excessive consumption of carbohydrates with a lack of dietary fiber contributes to the onset and progression of diabetes mellitus , especially with a hereditary predisposition to it. This is due to overload and then depletion of pancreatic cells, which produce insulin necessary for the absorption of glucose. Disorders of fat metabolism, characteristic of atherosclerosis, can also be triggered by excessive consumption of easily digestible carbohydrates, especially sucrose. So it’s much healthier to get the carbohydrates your body needs from vegetables, fruits, grains and legumes.
Starch
Starch is a polysaccharide built from cyclic α-glucose residues.
It includes:
- amylose (inner part of starch grain) – 10-20%
- amylopectin (starch grain shell) – 80-90%
The amylose chain includes 200 - 1000 α-glucose residues (average molecular weight 160,000) and has an unbranched structure.
Amylopectin has a branched structure and a much larger molecular weight than amylose.
Properties of starch
- Hydrolysis of starch : when boiled in an acidic environment, starch is hydrolyzed successively:
Recording the complete hydrolysis of starch without intermediate steps:
- Starch does not give a “silver mirror” reaction and does not reduce copper (II) hydroxide.
- Qualitative reaction to starch: blue color with iodine solution.
Disorders of carbohydrate metabolism in children
The peculiarities of metabolism and nutrition of newborns lead to the fact that glycolysis in their bodies is 30% more intense than in an adult. Therefore, it is important to determine the causes of carbohydrate metabolism disorders in a baby. After all, the first days of a person are filled with events that require a lot of energy: birth, stress, increased physical activity, food consumption, breathing oxygen. Glycogen levels normalize only after a few days.
In addition to hereditary diseases related to metabolism, which can appear from the first days of life, a child is susceptible to a variety of conditions that can lead to celiac disease. For example, an upset stomach or small intestine.
In order to prevent the development of celiac disease, the level of glucose in the baby’s blood is studied during the period of intrauterine development. That is why the expectant mother must undergo all tests prescribed by the doctor and undergo instrumental examinations during pregnancy.
What are carbohydrates?
Carbohydrates are a large group of substances that mainly consist of hydrogen, oxygen and carbon. Some complex carbohydrates also contain sulfur and nitrogen.
All living organisms on our planet are composed of carbohydrates. Plants consist of almost 80% of them, animals and humans contain much less carbohydrates. Carbohydrates are mainly found in the liver (5-10%), muscles (1-3%), and brain (less than 0.2%).
We need carbohydrates as a source of energy. With the oxidation of just 1 gram of carbohydrates, we get 4.1 kcal of energy. In addition, some complex carbohydrates are storage nutrients, and fiber, chitin and hyaluronic acid give tissue strength. Carbohydrates are also one of the building blocks of more complex molecules such as complex proteins, nucleic acid, glycolipids, etc. Without the participation of carbohydrates, the oxidation of proteins and fats is impossible.
Differences between glucose and fructose metabolism
The metabolism of fructose, which has a different structure from glucose, occurs somewhat differently, so the following factors must be taken into account:
- Fructose is the only available source of fast carbohydrates for people suffering from diabetes.
- The glycemic load of fruits is lower than that of any other food. For example, watermelon is one of the sweetest and largest fruits and has a glycemic load of about 2. This means that per kilogram of watermelon there are only 20 grams of fructose. To achieve the optimal dosage at which it will be converted into adipose tissue, you need to eat about 2.5 kilograms of this sweet fruit.
- Fructose tastes sweeter than sugar, which means that by using sweeteners based on it, you can consume fewer carbohydrates overall.
Now let’s look at the differences between the metabolism of carbohydrates to fructose and glucose, respectively.
| Glucose metabolism | Metabolism of fructose |
| Part of the incoming sugar is absorbed in the liver cells. | Practically not absorbed in the liver. |
| Activates the insulin response. | .In the process of metabolism, alkaloids are released that poison the body. |
| Activates the glucagon reaction. | They do not participate in the transition of food sources to external sugar. |
| It is the body's preferred source of energy. | They pass into adipose tissue without the participation of insulin. |
| Participates in the creation of glycogen cells. | They cannot participate in the creation of glycogen reserves due to their more complex structure and completed form of the monosaccharide. |
| Low sensitivity and possibility of conversion to triglycerides. | Highly likely to be converted into fatty tissue with relatively little consumption. |